Chargeability
Chargeability is a physical property related to conductivity. As we learned previously, ionic charges within a rock’s pore water begin to move under the influence of an electric field, resulting in electrical current. However, some of the pore ions do not move uninhibited through the rock and begin to accumulate at impermeable boundaries. This build-up of ionic charges is commonly referred to as induced polarization (IP), as it is responsible for generating electric dipole moments within the rock. We use chargeability to characterize the formation and strength of the induced polarization within a rock, under the influence of an electric field.
The physical explanation for causes of chargeabilty are complex and not completely understood. Certainly the effects are dependent upon the microscopic nature the material and specifically upon the surface to volume ratio of pore material and the types of fluids in the rock. The two images below offer some insight into the complexity.
Despite the complexity are two primary phenomenological mechanisms which are insightful in characterizing the chargeable behaviour of rocks: membrane polarization and electrode polarization.
Membrane Polarization
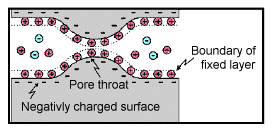
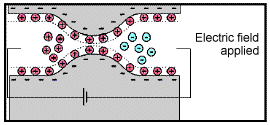
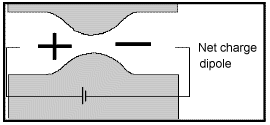
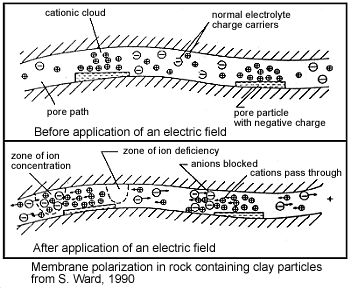
Membrane polarization occurs when the pore space narrows to a within several ion widths.
Because ionic charges cannot be forced through the pore throat, they accumulate on either side when an electric field is applied; with positive charges accumulating on one side of the pore throat and negative charges accumulating on the other. The accumulation of charges eventually stops because the electric fields from the blocked charges becomes large enough that it prevents other ions of the same sign from joining the group.
The net separation of positive and negative charges across narrow pore spaces generates a set of electric dipole moments which is ultimately responsible for the voltages measured in induced polarization survey.
Electrode Polarization
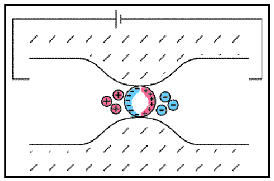
Electrode polarization occurs when the pore space is blocked by metallic particles. When an electric field is applied, the metallic particles become electrically charged and attract nearby ions.
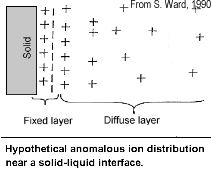
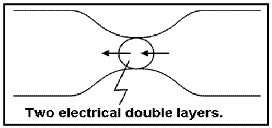
The attraction of the ions to the surface forms a primary layer of fixed ionic charges, followed by a secondary diffuse layer of opposing charges. This is known as an electric double layer.
Each electric double layer results in an electric dipole moment which contributes towards the induced polarization within the rock.
Chargeabilities of Common Rocks
Tables (from Telford et al, 1976) provide a very general guide to the integrated chargeabilities of materials. Because different intervals of integration \([t_1,t_2]\) are used for each table, chargeability values cannot be compared between tables. However, we can infer several things from these tables:
The individual properties of rocks results in a variation in chargeability (click here for table).
Chargeability increases as the % abundance of sulphide minerals increases (click here for table).
Highly porous rocks such as extrusive volcanics and sandstones are more chargeable than hard rocks such as granites and limestones (click here for table).
The type of ore-mineralization impacts the chargeability of rocks to varying degrees (click here for table).
Factors Impacting Chargeability
Sulphide Mineralization:
As we discussed earlier, electrode polarization occurs when the pore path is blocked by metallic particles. A major source of these metallic particles is sulphide mineralization. As the abundance of sulphide minerals within a rock increases, so does the electrode polarization. Therefore, highly mineralized rock tend to be very chargeable.
Clays:
Clays have a tendancy to partially block the path which ions take through the rock’s pore water. Upon application of an electric potential, positive charge carriers pass easily, while negative carriers accumulate. This results in an “ion-selective” membrane polarization. Clays represent a dominant source of induced polarization in unmineralized sedimentary rocks.
A surplus of both cations and anions occurs at one end of the membrane, while a deficiency occurs at the other end. The reduction of mobility is most obvious at frequencies slower than the diffusion time of ions between adjacent membrane zones; i.e. slower than around 0.1 Hz. Conductivity increases at higher frequencies.
Pore-Water Salinity:
The induced polarization within a rock depends on having a mechanism for accumulating ionic charges. It also depends on the salinity of the pore water; i.e. the concentration of ions within the pore water. As the pore-water salinity increases, so does the capacity of the rock to support a build-up of ionic charges. This results in an increases chargeability for the rock.
Tortuosity:
Tortuosity defines the connectivity and complexity of a rock’s pore-space network. As the tortuosity of the rock’s pore-space increases, it becomes more difficult for ionic charges to move through the rock. As a result, and increases abundance of ionic charges will accumulate within the rock when it is subjected to an electric field. Thus, the chargeability of a rock increases and its tortuosity increases.